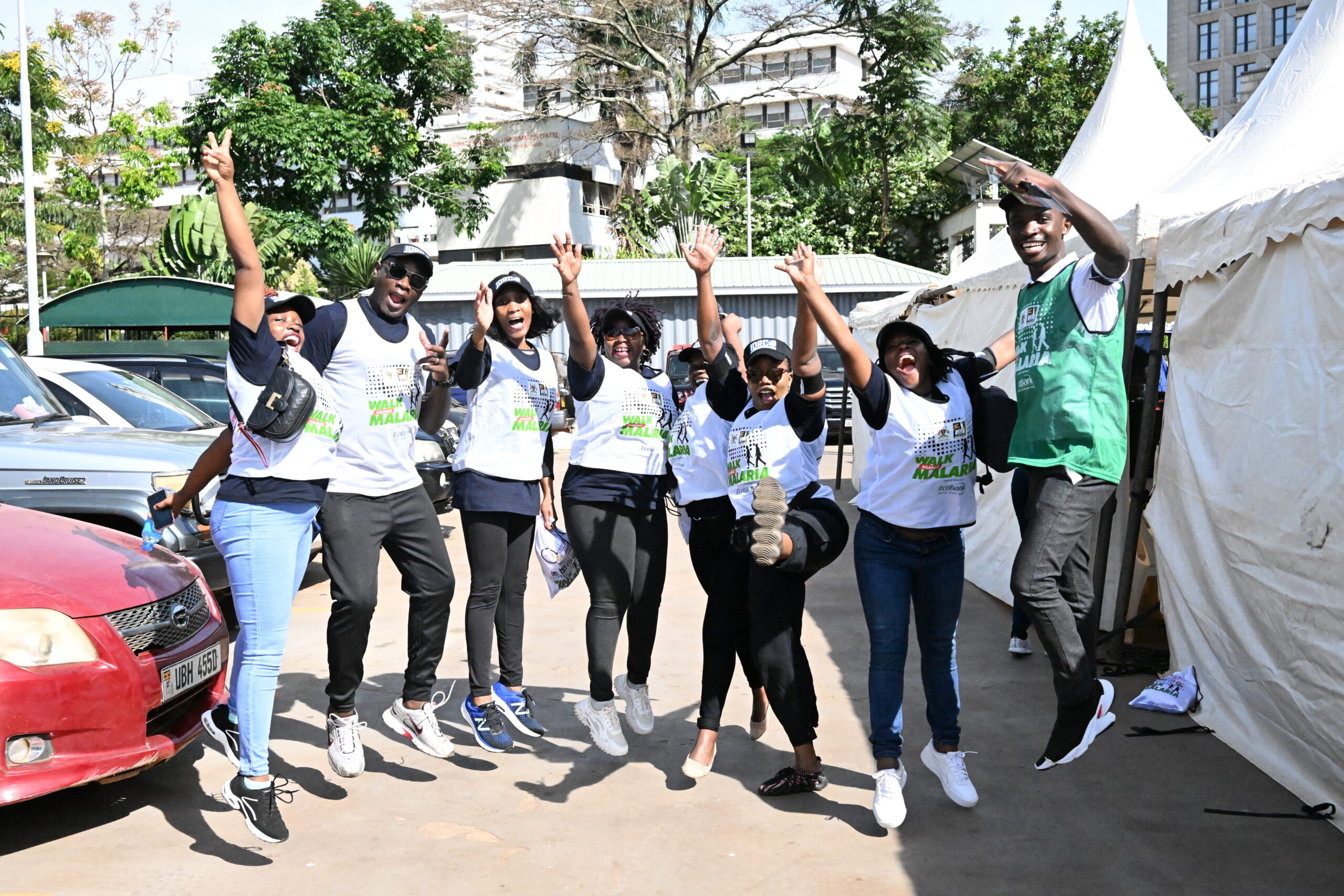What We Do
Infectious diseases research
IDRC conducts high quality research in infectious and other diseases of public health importance through collaboration and partnerships. Our key research areas include malaria, Tuberculosis and HIV.


Malaria Research

Tuberculosis Research

HIV Research

Growing With Our Clients
25 Years Of Your Trust
Leverage agile frameworks to provide a robust synopsis for high level overviews. Iterative approaches to corporate strategy foster collaborative thinking to further the overall value proposition.
Meet Avantage Professionals
Commited To Your Business
Given the complexity of forming a team including consciously or unconsciously developing team interaction norms and guidelines, ending up with an effective, functioning team is downright amazing.

Dedicated To Your Business
Better Business With Avantage


We are social


Excellence in infectious diseases research
Our Impact
We prioritize the dissemination of our research findings through multiple relevant channels as part of our commitment to increasing knowledge and understanding in the field of infectious diseases. We have produced over 500 publications as a result of our rigorous research efforts, including policy briefs and articles published in prestigious peer-reviewed journals. These publications are excellent resources for policymakers because they provide trustworthy data, intelligent analysis, and evidence-based recommendations.


Excellence in infectious diseases research
Our Impact
Our studies have led to over 500 publications and have impacted on the management of infectious diseases in Uganda. IDRC currently manages over 47 grants. IDRC was established by Uganda health scientists from Makerere University College of Health Sciences and the Uganda Ministry of Health.
012345678900123456789001234567890+
Publications
0123456789001234567890+
Grants
0123456789001234567890+
Years of Service

0123456789001234567890%
Client Success
012345678900123456789001234567890+
Businesses Advised
012345678900123456789001234567890+
Guides Given
0123456789001234567890+
Awards Achieved
Our Offices
Let's Talk
Come and visit our quarters or simply send us an email anytime you want. We are open to all suggestions from all clients, old or new.
San Diego
411 D Avenue, San Diego, CS 91950
London
Bloomsbury Square, London WC1B 4EA
Email us
info@avantage.com
office@avantage.com
office@avantage.com
Call us
619 270 8578
619 270 8879
619 270 8879
Request a Call Back
If you’d like to talk to our consulting team, contact us via the form and we’ll get back to you shortly.